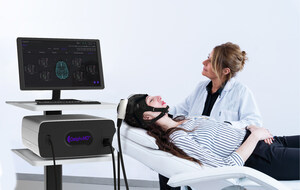
With CE MDR approval, QuantalX is set to scale the commercialization of its accessible and objective neurodiagnosis test across Europe. The Delphi-MD device will be deployed in neurological care centers, neurology departments, and brain health networks. Delphi-MD will streamline improved brain health assessment, early detection and differential diagnosis of any brain abnormality or disease such as stroke, Parkinson disease, dementia, Alzheimer's disease, Normal Pressure Hydrocephalous (NPH) and more, as well as prediction of individual patients' treatment response.
The aging population in Europe is driving a sharp rise in neurodegenerative diseases such as dementia, Parkinson's, and stroke. According to the World Health Organization (WHO), caring for the 14.1 million people living with dementia in Europe cost $439 billion in 2019, averaging $31,144 (€27,815) per patient. Parkinson's disease, the fastest-growing neurological disorder, affects over 1.2 million people across Europe, with cases expected to rise as the population ages. By 2050, the number of Europeans aged 60 and older is projected to increase significantly, further straining healthcare systems and highlighting the urgent need for advanced care solutions.
Dr. Iftach Dolev, CEO and Co-founder of QuantalX, shared his thoughts on this landmark achievement: "CE MDR approval validates the unparalleled innovation behind our neurodiagnosis test. It's a testament to our team's dedication and marks the beginning of a new era in brain health diagnostics."
With its ability to enable early detection and precise diagnosis of neurological disorders, Delphi-MD will play a crucial role in improving patient outcomes and quality of life while alleviating the growing economic and clinical burden on Europe's healthcare systems.
About QuantalX Neuroscience
QuantalX is committed to fundamentally improving patient care and alleviating the burden on healthcare systems through objective and accurate, early detection and differential diagnosis of brain abnormalities, leveraging its novel Direct Neuro-Physiological technology, the Delphi-MD device.
Media Contact:
Adi Jacobson
VP Marketing, QuantalX Neuroscience
[email protected]
Tel: +972502043934
Photo - https://mma.prnewswire.com/media/2617703/QuantalX_Delphi_MD.jpg
SOURCE QuantalX






Share this article