Pulsenmore Receives Brazilian ANVISA Approval for Its Prenatal Home Ultrasound Solution
RAMAT GAN, Israel, Jan. 10, 2024 /PRNewswire/ -- Pulsenmore (TASE: PULS), the global leader in self-scan ultrasound technology for home use, proudly announces the approval of its pioneering prenatal care solution by the Brazilian Health Regulatory Agency (ANVISA), marking a significant milestone in the company's international expansion.
This endorsement positions Pulsenmore at the forefront of innovative healthcare, unlocking possibilities for remote prenatal care across Brazil and paving the way for expansion into multiple Latin American countries. The approval underscores Pulsenmore's commitment to transforming the landscape of prenatal care, offering a groundbreaking solution for expectant mothers and healthcare organizations alike.
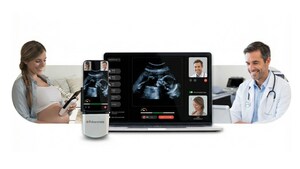
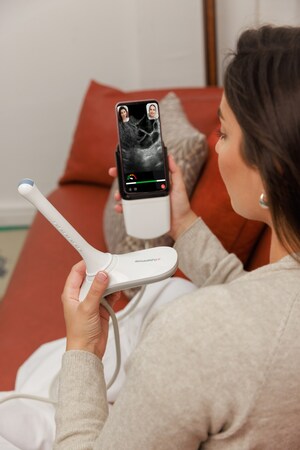
Pulsenmore's revolutionary home ultrasound technology empowers pregnant women to conduct ultrasound imaging scans from the comfort of their homes, using their personal smartphones connected to a dedicated device and application. These scans are seamlessly transmitted to remote hospitals or clinics for evaluation, focusing on crucial fetal vitality parameters.
The approval from ANVISA now enables Pulsenmore to provide Brazilian health organizations and consumers with unparalleled access to this transformative solution, reducing the necessity for in-clinic visits and offering a continuous, personalized healthcare experience. Clinicians can engage with patients in real-time or asynchronously, promoting increased patient satisfaction and efficient resource management.
With approximately 2.6 million live births, Brazil faces unique challenges in its healthcare landscape. However, the pandemic has accelerated the digitalization of healthcare, making Telemedicine an integral part of the healthcare landscape. The recently approved General Telemedicine Law further supports innovative solutions like Pulsenmore, fostering accessibility and convenience in healthcare.
Pulsenmore's solution aligns seamlessly with the evolving healthcare needs of the country, especially in addressing high-risk pregnancies, premature births and access in remote regions.
Dr. Elazar Sonnenschein, CEO and Founder of Pulsenmore, stated, "Our proven remote monitoring solution enables patients to maintain their routines while receiving the best standard of pregnancy care. We are excited about the positive impact Pulsenmore can bring to Brazilian healthcare, ushering in a new era of accessible and high-quality prenatal care."
For more information please visit www.pulsenmore.com
Press Contact:
Mira Altmark Sofer
+972505911212
VP Marketing
Pulsenmore
Video - https://www.youtube.com/watch?v=gzILwjkmshg
SOURCE Pulsenmore Ltd.






Share this article