
〜Driving Technology Transfer and Test Manufacturing Following Regulatory Approval to Launch Company-Led af-001 Trial〜
TOKYO, Oct. 8, 2025 /PRNewswire/ -- Alpha Fusion Inc. (Alpha Fusion) is proud to announce a joint initiative with the Kobe City Medical Center General Hospital (KCGH) to establish a domestic supply system for investigational drugs utilizing Astatine-211 (At-211). This collaboration significantly advances the foundational work for what is expected to be the world's first company-sponsored clinical trial using At-211 (as of September 2025).
KCGH has secured usage approval for At-211 from Nuclear Regulation Authority. The hospital is now proceeding with technology transfer and test manufacturing of the investigational drug for Alpha Fusion's lead pipeline, af-001 (targeting differentiated thyroid cancer), in preparation for clinical trial initiation. This pioneering effort is setting up a system to seamlessly translate At-211 drug discovery into clinical practice.
Pioneering the World's First Company-Led At-211 Investigational Drug Supply Chain
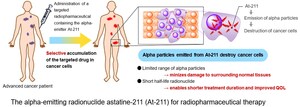
At-211 is recognized as an exceptionally promising radionuclide for targeted alpha therapy due to its short half-life (approx. 7.2 hours) and simple decay properties. However, a stable supply system for the investigational drug has been a major global hurdle, resulting in all clinical trials to date being academia-led.
In a breakthrough, the partnership with KCGH has established the crucial supply chain necessary to conduct clinical trials in Japan. KCGH operates a radioactive investigational drug GMP manufacturing facility. The hospital's extensive experience in manufacturing investigational drugs using short-lived radionuclides for PET (Positron Emission Tomography) will be adapted for At-211 formulations. Establishing this supply system represents a landmark achievement for global therapeutic development using At-211.
Alpha Fusion has already formed a network with Japanese academic cyclotron facilities capable of At-211 production. By incorporating the hospital's GMP manufacturing facility into the system, Alpha Fusion is building the world's first multi-site supply chain for an At-211 radiopharmaceutical, connecting radionuclide production, drug manufacturing, and clinical trial sites.
af-001: The Lead Pipeline Harnessing At-211's Unique Properties
Utilizing the collaborative framework with KCGH, Alpha Fusion is preparing to launch its first company-led trial with its lead pipeline, af-001. This program targets differentiated thyroid cancer. It exploits At-211's unique property—shared with iodine—of being taken up by the Sodium-Iodide Symporter (NIS), allowing the drug to selectively accumulate in cancer cells. This specific approach is only feasible with At-211 among alpha-emitting radionuclides. Furthermore, the low surrounding radiation risk associated with At-211 is expected to allow for outpatient treatment, offering a powerful and highly convenient new therapeutic option for thyroid cancer patients.
This advancement solidifies the company's leading global position as a "clinical-stage At-211 drug discovery company".
Comment from Sunao Fujioka, CEO of Alpha Fusion:
"Targeted alpha therapy with At-211 could become a new cornerstone of cancer treatment, yet supply has been the global bottleneck. By partnering with KCGH to launch this investigational drug supply system, we have solved this key challenge ahead of the world, marking a giant leap toward clinical implementation. We are accelerating both our clinical development and supply efforts to deliver this new treatment to patients as quickly as possible."
Comment from Tomohiko Yamane, Director of Department of Molecular Imaging Research, KCGH:
KCGH has, over the past decade, manufactured in-house investigational PET diagnostic radiopharmaceuticals, supporting numerous clinical trials, including those for Alzheimer's disease treatments. In recent years, the use of radiopharmaceuticals has expanded from diagnostics to cancer treatment. We regard our participation in Alpha Fusion's company-led trial as a significant step forward in applying our manufacturing and quality-control expertise in radiopharmaceuticals to therapeutic applications. From Kobe, we are committed to delivering new treatment options to patients in our community and around the world as swift as possible."
Future Outlook
The valuable expertise gained by Alpha Fusion in technology transfer, investigational drug manufacturing, and logistics for At-211 therapeutics can be applied to future overseas clinical trials and commercial supply chain design. The successful system established in Japan will serve as a model case for replication and expansion in international markets, starting with the U.S. We are already in active discussions with domestic and international partners to manage these future supply chains.
Alpha Fusion plans to launch the clinical development of multiple At-211 pipelines both domestically and internationally, beginning with af-001. With the domestic investigational drug supply system now in place, we will accelerate the international rollout of At-211 drug discovery, aiming to create medicines for patients currently without treatment options.
SOURCE Alpha Fusion Inc






Share this article