Biohaven Announces Robust Clinical Data with Single Dose Rimegepant That Defines Acute and Durable Benefits to Patients: The First Oral CGRP Receptor Antagonist to Deliver Positive Data on Pain Freedom and Most Bothersome Symptom in Two Pivotal Phase 3 Trials in Acute Treatment of Migraine
- Broad and clinically important benefits beyond the initial registrational endpoints are now reported.
- Durability of clinical effect was seen across multiple outcome measures and findings were consistent across both pivotal Phase 3 trials.
- A single dose of rimegepant, without any rescue medications, was superior to placebo for pain freedom and pain relief at 2 hours post-dosing, and showed a profile of increasing improvement throughout the first eight hours that was sustained compared to placebo out to 24 and 48 hours.
- The vast majority of rimegepant treated patients did not take rescue medications during the 24 hour period after dosing.
- Rimegepant-treated patients showed improvement on measures of functional disability with a greater proportion of patients achieving normal function.
- Rimegepant demonstrated a safety profile similar to placebo including liver function tests.
- Tolerability profile with rimegepant was similar to placebo and favorable compared to historical triptan experience.
- More than 3x the number of subjects who responded to treatment preferred rimegepant over their previous therapy.
LOS ANGELES, April 22, 2018 /PRNewswire/ -- Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN) today announced the results of key secondary outcome measures from Phase 3 clinical trials (BHV3000-301 and BHV3000-302) of rimegepant, the first oral CGRP receptor antagonist to deliver positive data on pain freedom and most bothersome symptom in two pivotal Phase 3 trials in acute treatment of migraine. As previously reported, rimegepant met the co-primary efficacy endpoints with significant superiority to placebo, at two hours post-dose, on pain freedom and freedom from the most bothersome symptom (MBS); endpoints which, according to FDA guidance, are needed for registrational filing. These clinical trials showed an early separation from placebo and a profile of continued improvement after a single dose of rimegepant in patients who did not take any rescue medications.

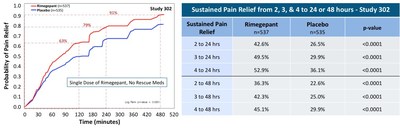
A durable effect with rimegepant was achieved with pain freedom lasting up to 48 hours following a single dose. The percent of patients experiencing pain freedom between 0 to 8 hours after dosing is depicted in the Kaplan-Meier curves in Figure 1. The percent of patients experiencing sustained pain freedom from 2 to 24 hours and through 2 to 48 hours is shown in the accompanying table. Increasing benefit is evident from 2 to 4 hours with durability of these effects lasting through 48 hours.
Figure 1: Durable Treatment Effects on Pain Freedom1
Approximately 90% of patients who took rimegepant and no rescue medications achieved the clinically important endpoint of pain relief within 8 hours, which signifies an important return to daily function (Figure 2). Onset of pain relief was observed early with numerical separation evident approximately 30-45 minutes post-dosing. By 90 minutes statistical significance was achieved in both studies. Pain relief was sustained throughout 48 hours (Figure 2). The sustained pain relief profile was similar to that observed for sustained pain freedom.
Figure 2: Durable Treatment Effects on Pain Relief2
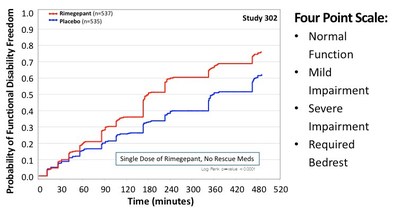
Pain relief is associated with reduced disability and function as shown in these studies (Figure 3). Rimegepant-treated patients showed improvement on functional disability at 2 hours post-dosing in both pivotal trals. This outcome is clinically important because it indicates the patient's recovery and ability to resume daily activities.
Figure 3: Freedom from Functional Disability (Patients Achieving Normal Function)3
Vlad Coric, M.D., Chief Executive Officer of Biohaven, commented, "We are proud to be the first to deliver a positive dataset from two Phase 3 pivotal trials meeting current FDA guidance that shows robust and durable clinical benefit from a single dose of an oral CGRP receptor antagonist in the acute treatment of migraine. The strength of these data are highlighted by rimegepant showing benefits over placebo in 11 out of 13 prespecified primary and secondary outcome measures in both trials. Our observations in both trials of comprehensive and durable efficacy, a favorable safety and tolerability profile, and the ease of oral dosing, together represent an attractive profile to submit to regulatory authorities with the goal of ultimately bringing relief to patients suffering from migraine."
Richard B. Lipton, M.D., Vice Chair of Neurology, Professor of Epidemiology and Population Health and Director of the Montefiore Headache Center, at the Albert Einstein College of Medicine, and Chair of Biohaven's CGRP Scientific Advisory Board added, "The single dose data from these two clinical trials are very exciting as rimegepant brings forward a novel mechanism of action that has demonstrated important clinical benefit to patients. Most of the patients I see with migraine are actively working and raising children, and relieving pain and restoring function means they can once again take care of their families and return to work. The new data reviewed today encourages me that rimegepant, if approved, can meet their needs for an acute treatment with lasting clinical benefit."
Over 36 million Americans suffer from migraine. Acute attacks of migraine can differ in intensity and frequency, with many being highly disabling. More than 90% of migraine sufferers are unable to work or function normally during an attack. CGRP receptor antagonists represent a novel class of drug candidates for the treatment of migraine and are the first new class specific to the acute treatment of migraine in over 25 years. This unique and specific mode of action potentially offers an alternative to current agents, particularly for those who have contraindications to the use of triptans, such as patients with underlying cardiovascular diseases, or for patients who do not respond to triptans.
For full presentation including use of rescue medications, patient preference, safety summary and other secondary outcome measures see the Events page on the company website (see link below).
Conference Call and Webcast
Biohaven hosted a conference call and webcast on April 22, 2018 concurrent with the American Academy of Neurology meeting in Los Angeles to discuss the secondary outcome measures available from these two Phase 3 clinical trials. To access the audio webcast with slides, please visit the "Events" page in the Investors section of the Company's website at http://investors.biohavenpharma.com/events. An archive of today's teleconference and webcast will be available for 6 months following the call.
About Biohaven
Biohaven is a clinical-stage biopharmaceutical company with a portfolio of innovative, late-stage product candidates targeting neurological diseases, including rare disorders. Biohaven has combined internal development and research with intellectual property licensed from companies and institutions including Bristol-Myers Squibb Company, AstraZeneca AB, Yale University, Catalent, Rutgers, ALS Biopharma LLC and Massachusetts General Hospital. Currently, Biohaven's lead development programs include multiple compounds across its CGRP receptor antagonist and glutamate modulation platforms. The company's common shares are listed on the New York Stock Exchange and traded under the ticker symbol BHVN. More information about Biohaven is available at www.biohavenpharma.com.
Forward-Looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements involve substantial risks and uncertainties, including statements that are based on the current expectations and assumptions of the Company's management. All statements, other than statements of historical facts, included in this press release, including the Company's timing of the expected NDA submission for rimegepant and its potential to be an improved treatment option for the acute treatment of migraine, are forward-looking statements. The use of certain words, including "believe" and "will" and similar expressions, is intended to identify forward-looking statements. The Company may not actually achieve the plans and objectives disclosed in the forward-looking statements, and you should not place undue reliance on the Company's forward-looking statements. Various important factors could cause actual results or events to differ materially from those that may be expressed or implied by our forward-looking statements, including that topline data is based on preliminary analysis of key efficacy and safety data, and such data could change following a more comprehensive review and evaluation of more extensive data from the trials that the Company has not yet received, and these preliminary conclusions may not accurately reflect the complete results of the clinical trials, and uncertainties relating to the timing for submitting an NDA and the potential regulatory approval of rimegepant. Additional important factors to be considered in connection with forward-looking statements are described in the "Risk Factors" section of the Company's Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 6, 2018 and other filings Biohaven makes with the U.S. Securities and Exchange Commission from time to time. The forward-looking statements are made as of this date and the Company does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
For further information, contact Dr. Vlad Coric, the Chief Executive Officer at [email protected]
SOURCE Biohaven Pharmaceutical Holding Company Ltd.
Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In
Share this article