
- The 300,000 prodisc® Total Disc Replacement (TDR) milestone achievement is supported by extensive long-term published evidence and validated across decades of clinical success, with a reported reoperation rate of less than 1%.1
- prodisc® technology leads the U.S. and international TDR markets through exceptional long-term clinical outcomes, continuous cervical and lumbar TDR product innovation, and recent expanded patient indications.2
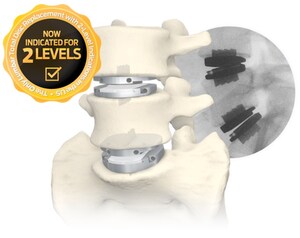
- Most recently, Centinel Spine received FDA Premarket Approval in October 2025 for two-level use of prodisc C Vivo and prodisc C SK cervical TDR devices, expanding total disc replacement treatment options.
- Centinel Spine's prodisc is the only TDR solution in the U.S. with FDA-approved two–level indications for both cervical and lumbar use.
WEST CHESTER, Pa., Nov. 13, 2025 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced that more than 300,000 prodisc devices have now been implanted worldwide. This milestone achievement underscores over three decades of clinical performance, surgeon trust, and continued expansion of total disc replacement solutions. The prodisc cervical and lumbar technologies will be highlighted by Centinel Spine at the upcoming 2025 North American Spine Society (NASS) 40th Annual Meeting in Denver, Colorado, November 14-16, 2025.
The prodisc platform remains the only total disc replacement system offering multiple anatomically tailored implant solutions for both the cervical and lumbar spine, empowering surgeons to Match-The-Disc™ to each patient's unique anatomy and clinical needs. The design and clinical durability of prodisc have been supported by more than 540 published peer reviewed papers, with long-term evidence demonstrating sustained function and less than 1% revision rates.1
Reaching 300,000 prodisc implantations is a historic milestone demonstrating long-term clinical success and device durability; however, Centinel Spine continues to aggressively innovate and advance the field of total disc replacement. Recent achievements include FDA approval for two-level indications of the prodisc L Lumbar TDR device; the introduction of Anatomic Endplate™ versions and new instrumentation for the prodiscL system; the launch of the prodisc C Vivo and prodisc C SK Cervical Match-the-Disc systems; and the limited commercial release of the prodisc C Nova cervical TDR device. Most recently, the Company received FDA approval for two-level use of the prodiscC Vivo and prodisc C SK cervical TDR devices. Centinel Spine remains exclusively dedicated to total disc replacement, with extensive and continuous innovation planned well into the future.
"prodisc has changed functional outcomes for my own patients over the past two decades. While this milestone of 300,000 implantations is meaningful, we cannot lose sight of how this advanced surgical technology will continue to shape the future management of patients with degenerative spine conditions for many years," said Michael E. Janssen, DO of the Center for Spine & Orthopedics in Denver, Colorado.
"A fixed core center-of-rotation prosthesis, such as prodisc, provides spinal stability and healthy mobility in the hands of experienced surgeons. Maintaining controlled motion supports reduced mechanical complications and long-term construct longevity. Reaching 300,000 implantations worldwide reflects long-term trust in the total disc replacement procedure," added A/Prof. Dennis Hey, MBBS, FRCS(Orth), Senior Consultant Spine Surgeon at National University Spine Institute, Clinical Director for Orthopaedic Surgery at National University Hospital, Head of Orthopaedic and Spine Surgery, and Founding Director of Motion-preserving and Minimally-invasive Spine Unit (MMSU) at Alexandra Hospital, Singapore.
As surgeon adoption expands globally and clinical data continues to mature, Centinel Spine remains committed to advancing total disc replacement for patients worldwide.
"Reaching 300,000 prodisc implantations is an important milestone reflecting the trust surgeons place in total disc replacement and the meaningful impact this technology has had on patients," concluded Steve Murray, CEO of Centinel Spine. "We remain focused on supporting surgeons, providing clinically proven solutions, and advancing total disc replacement as an important treatment option for patients with cervical and lumbar spinal disease."
1. Based upon U.S. complaint handling units for prodisc since launch in 2006.
2. Based on publicly available information and data on file as of November 2025.
About Centinel Spine, LLC
Centinel Spine®, LLC is the leading global medical device company exclusively focused on addressing cervical and lumbar spinal disease with prodisc®, the most complete total disc replacement (TDR) technology platform in the world.
The Company's prodisc technology is the most studied and clinically-proven TDR system across the globe, validated by over 540 published papers and more than 300,000 implantations. Centinel Spine's prodisc is the only TDR technology with multiple motion-preserving anatomic solutions, allowing the surgeon to Match-the-Disc™ to each patient's anatomy for both cervical and lumbar total disc replacement.
For more information, please visit the company's website at www.CentinelSpine.com or contact:
Varun Gandhi
Chief Financial Officer
900 Airport Road, Suite 3B
West Chester, PA 19380
Phone: 484-887-8871
Email: [email protected]
SOURCE Centinel Spine, LLC






Share this article