
EBR Systems Achieves First Enrollments in the WiSE-UP Study for Heart Failure Patients
The WiSE-UP post-approval study is gathering real-world evidence from commercially treated patients using the WiSE® left ventricular endocardial pacing (LVEP) system.
Key Highlights:
- The first two patients have now been enrolled in the WiSE-UP post-approval study by Dr Devi Nair from St Bernards Heart & Vascular Center, Arkansas
- The EBR-sponsored study will evaluate real-world outcomes for heart failure patients receiving the FDA-approved WiSE® System for delivering LVEP in patients unable to receive traditional cardiac resynchronization therapy (CRT)
- The study will follow more than 300 patients across 50 US centers over a five-year period, and will generate both short- and long-term performance metrics
SUNNYVALE, Calif., Nov. 7, 2025 /PRNewswire/ -- EBR Systems, Inc., developer of the world's only wireless cardiac pacing system for heart failure, today announced the first patient enrollments in the WiSE® System Utilization & Performance (WiSE-UP) Study. The first two patients were treated at St. Bernards Heart & Vascular Center in Arkansas by Dr. Devi Nair, a globally recognized electrophysiologist.
This milestone marks an important step forward in advancing cardiac resynchronization therapy (CRT) for patients with heart failure with the addition of LVEP.
Sponsored by EBR Systems, the WiSE-UP Study is a prospective, observational post-approval study designed to evaluate real-world outcomes in patients treated with the FDA-approved WiSE System, which delivers left ventricular endocardial pacing (LVEP) for CRT. The study will follow more than 300 patients across 50 centers over five years, generating both short- and long-term performance data to inform future clinical practice.
"It was an honor to lead the team performing the first WiSE System implant as part of the WiSE-UP Study," said Devi Nair, MD, FACC, FHRS, Electrophysiologist at St. Bernards Heart & Vascular Center. "Our entire team is proud to contribute to this important study, which we believe will further demonstrate the benefits of left ventricular endocardial pacing for patients with heart failure. We look forward to continuing our collaboration with EBR Systems as we work together to advance innovation in cardiac resynchronization therapy and improve patient outcomes."
Niraj Varma, MD, PhD, FRCP, Professor of Medicine at the Cleveland Clinic and the National Principal Investigator for the study said: "The launch of the WiSE-UP Study marks an exciting moment in cardiac resynchronization therapy. This study will evaluate the application of left ventricular endocardial pacing in real world practice, capture important clinical outcomes over time, and assess sustained therapeutic impact of the WiSE System. I am proud to lead this initiative that will continue to develop the standards of care for heart failure patients."
For more information about EBR, please visit https://www.ebrsystemsinc.com/.
About EBR Systems
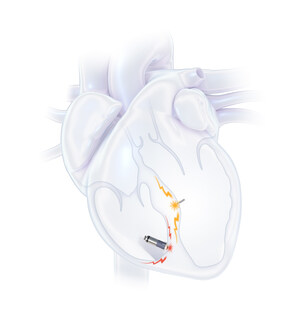
EBR Systems is a cardiac rhythm management company on a clear mission: to transform the lives of people with heart failure by helping physicians deliver optimal cardiac pacing. The WiSE System—the first FDA approved system for leadless left ventricular endocardial pacing (LVEP) in CRT—combines advanced engineering with peer-reviewed clinical evidence to expand access for patients who previously lacked options and to improve outcomes.
About the WiSE Technology
The WiSE System delivers LV endocardial pacing without a transvenous LV lead using targeted ultrasound. A subcutaneous transmitter and battery detect the RV pacing signal and, within milliseconds, emit ultrasound energy that a rice-sized endocardial electrode converts into an electrical pulse—enabling synchronized biventricular pacing. This LVEP approach is engineered for versatility across patient anatomies and is supported by peer-reviewed outcomes from SOLVE-CRT pivotal trial. WiSE is approved for commercial use in the United States and limited to clinical study use outside of the United States.
Forward-Looking Statements
This announcement contains or may contain forward-looking statements that are based on management's beliefs, assumptions, and expectations and on information currently available to management. Forward-looking statements involve known and unknown risks, uncertainties, contingencies and other factors, many of which are beyond the Company's control, subject to change without notice and may involve significant elements of subjective judgment and assumptions as to future events which may or may not be correct.
All statements that address operating performance, events or developments that we expect or anticipate will occur in the future are forward-looking statements, including without limitation our expectations with respect to our ability to commercialize our products and achieve broad market adoption including our estimates of potential revenues, costs, profitability and financial performance; our ability to develop and commercialize new products; our expectations with respect to our clinical trials, including enrollment in or completion of our clinical trials and our associated regulatory applications and approvals; our expectations with respect to the integrity or capabilities of our intellectual property position. These forward-looking statements are based on EBR Systems' current expectations and inherently involve significant risks and uncertainties. EBR Systems' actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of certain risks and uncertainties including those risks described in more detail in its most recently filed Annual Report on Form 10-K, Quarterly Report on Form 10-Q, and other documents on file with the SEC from time to time and available on the SEC's website at www.sec.gov.
Management believes that these forward-looking statements are reasonable as and when made. You should not place undue reliance on forward-looking statements because they speak only as of the date when made. EBR does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. EBR may not actually achieve the plans, projections or expectations disclosed in forward-looking statements, and actual results, developments or events could differ materially from those disclosed in the forward-looking statements.
Foreign Ownership Restriction
EBR's ASX-traded (ASX: EBR) CHESS Depositary Interests (CDIs) are issued in reliance on the exemption from registration contained in Regulation S of the US Securities Act of 1933 (Securities Act) for offers or sales which are made outside the US. Accordingly, the CDIs have not been, and will not be, registered under the Securities Act or the laws of any state or other jurisdiction in the US. The holders of EBR's CDIs are unable to sell the CDIs into the US or to a US person unless the re-sale of the CDIs is registered under the Securities Act or an exemption is available. Hedging transactions with regard to the CDIs may only be conducted in accordance with the Securities Act.
SOURCE EBR Systems







Share this article