
New FORCE Study on Partial REBOA in Battlefield Conditions Aims to Improve Combat Casualty Care
U.S. Army Medical Research and Development Command Funds FORCE Study to Evaluate Advanced Hemorrhage Control Technology on the Battlefield - Prytime Medical
SAN ANTONIO, Nov. 1, 2024 /PRNewswire/ -- The U.S. Army Medical Research and Development Command has announced a new study that will assess the efficacy of the new 2+ hour pREBOA-PRO™ catheter in treating casualties in extremely far forward austere combat environments. The FORCE (Field Observation of REBOA in Combat Environments) study, officially titled A Study of Partial REBOA Use on the Battlefield: Enabling Transport of Combat Casualties with Non-compressible Torso Hemorrhage (NCTH), focuses on the urgent need to manage severe hemorrhage on the battlefield. The study utilizes the pREBOA-PRO™ catheter with the goal of providing longer partial aortic occlusion time to enable triage, treatment and transport for the critically injured. The study commenced September 2024. This effort was awarded through MTEC solicitation MTEC-24-01-MPAI and is funded by the DOD Combat Casualty Research Program (CCCRP) in accordance with the Congressional direction to establish military medical partnerships with the Ukraine specified in NDAA 2023 and 2024. To date, the CCCRP has funded research projects within Ukraine related to traumatic brain injury, hemorrhage control, traumatic stress, rehabilitation, wound management, severe sepsis, and trauma registry development. The DOD CCRP seeks to develop requirements driven knowledge and material solutions for the care of combat related traumatic injury during current and future conflicts.
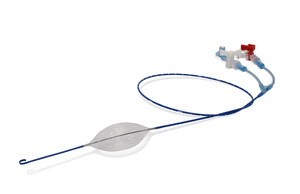
Non-compressible torso hemorrhage (NCTH) is the leading cause of preventable death among military trauma patients. In the current conflict in Ukraine, evacuation delays are common and require much longer hemorrhage control and resuscitation support than in previous conflicts. The pREBOA-PRO™ catheter, a third-generation device approved by the U.S. Food and Drug Administration and the European Union Medical Device Regulation – Regulation (EU) 2017/745 (EU MDR), has been in combat use in Ukraine for over 18 months. By extending the safe usage of resuscitative endovascular balloon occlusion of the aorta, or REBOA, beyond 30 minutes, the pREBOA-PRO™ catheter enables forward medical teams to hemodynamically stabilize critically injured soldiers outside of the hospital, improving their chances of survival during extended transport. This new catheter provides a critical solution by allowing over two hours of hemorrhage control and resuscitation support through a capability known as partial occlusion of the aorta. Its patented technology has become pivotal for casualty management on the modern battlefield.
Based on 18 months of successful use of the pREBOA-PRO™ in combat, the FORCE study will formalize the detailed collection of observational data on the use of the new 2+ hour partial REBOA pREBOA-PRO™ catheter in extremely austere and far forward combat environments. The key objectives of the study are to determine the utility of central aortic pressure monitoring and 2+ hour extended aortic occlusion on the battlefield.
REBOA has a long-standing history of military research & funding. Most recently, Prytime was selected by the U.S. Army Medical Research and Development Command to receive $6.2 million from the U.S. Department of Defense, awarded through the Medical Technology Enterprise Consortium for a multi-center observational study of NCTH patients being treated with the pREBOA-PRO for partial occlusion as standard of care. This effort is titled the Partial REBOA Outcomes Multi-Center Prospective (PROMPT) study scheduled to be completed November 30, 2025.
"No one should bleed to death: that is our company's mission," said Dr. Jessica Raley, the Principal Investigator for the FORCE and PROMPT studies. "We're passionate about finding new ways for civilian and combat wounded patients to reach definitive care, and we're grateful for the opportunity to document the latest REBOA combat casualty management strategies and share them with the world."
ABOUT PRYTIME MEDICAL DEVICES, INC.
Prytime Medical Devices, Inc. is an innovative medical device company that designs, develops, and commercializes minimally invasive solutions for hemorrhage control. The underlying intellectual property for REBOA was conceived based on lessons learned in war. Our latest innovations, the industry-leading ER-REBOA™ PLUS, and the innovative partial REBOA pREBOA-PRO™ catheter, enable torso hemorrhage control with more controlled resuscitation in a much wider range of clinical scenarios. The pREBOA-PRO™ catheter is the first and only FDA-cleared REBOA catheter designed specifically for True Partial REBOA™. More information can be found at http://www.PrytimeMedical.com.
ABOUT THE U.S. ARMY MEDICAL RESEARCH AND DEVELOPMENT COMMAND
The U.S. Army Medical Research and Development Command is the Army's medical materiel developer, with responsibility for medical research, development, and acquisition. USAMRDC produces medical solutions for the battlefield with a focus on various areas of biomedical research, including military infectious diseases, combat casualty care, military operational medicine, medical chemical and biological defense. https://mrdc.amedd.army.mil/
ABOUT MTEC
The Medical Technology Enterprise Consortium is a 501(c)(3) biomedical technology consortium that is internationally dispersed, collaborating with multiple government agencies under a 10-year renewable Other Transaction Agreement with the U.S. Army Medical Research and Development Command. The consortium focuses on the development of medical solutions that protect, treat, and optimize the health and performance of U.S. military personnel and civilians. To find out more about MTEC, visit mtec-sc.org.
Disclaimer: The views and conclusions contained herein are those of the authors and should not be interpreted as representing the official policies or endorsements, either expressed or implied, of the U.S. Government.
Contact Information:
For more information about this study, please contact: [Jessica Raley] [Principal Investigator for the FORCE study] [Prytime Medical] [210-340-0116] [[email protected]]
Press Contact: [Nicole Calvert] [Marketing Manager for Prytime Medical] [210-340-0116] [[email protected]]
SOURCE Prytime Medical






Share this article