
CARMEL, Ind., Aug. 2, 2021 /PRNewswire/ -- ParaPRO, a specialty pharmaceutical company focused on innovative anti-parasitic treatments, today announced the availability of Natroba™ (spinosad) Topical Suspension, 0.9%, the first product (new drug application) for scabies to be approved by the U.S. Food and Drug Administration (FDA) in more than 30 years.1 Earlier this year, the company received FDA approval of its supplemental New Drug Application (sNDA) for Natroba as a targeted topical treatment for scabies infestations in adult and pediatric patients four years of age and older. Natroba has been approved for the topical treatment of head lice infestations since 2011 and is currently the most frequently prescribed treatment option.2,3
"There are known limitations with existing scabicides, and for more than 30 years, practitioners have had to rely on the same active pharmaceutical ingredients to treat scabies," said Bill Culpepper III, President of ParaPRO. "We are pleased to offer healthcare providers and their patients the first pharmacological advancement in scabies treatment in decades. Natroba is a targeted topical therapy that was shown to be highly effective and well-tolerated in the treatment of scabies following one application of the study drug."
Natroba's approval was based on the FDA's new criteria for a "complete cure" and included data from two phase 3, multi-center, randomized, double-blind, vehicle-controlled clinical trials in patients from 206 households (533 patients) in which the youngest household member (index subject) four years of age or older had an active scabies infestation.3-6
Since 1989, "cured" outcomes from scabicides approved by the FDA were based on investigator observation.7 In 2016, the FDA established new, objective criteria for determining a "complete cure" which is now defined as being both clinical and confirmatory. A scabies patient is considered cured if a practitioner determines that all signs and symptoms have completely resolved, including burrows, inflammatory/non-inflammatory lesions, and pruritus, and conducts microscopic or dermatoscopic assessments to validate the absence of mites, eggs, scybala and burrows.8
"The approval of Natroba's expanded indication is a significant advancement in the scabies treatment landscape," said Dr. Anthony J. Mancini, Professor of Pediatrics and Dermatology, Northwestern University Feinberg School of Medicine and Head of Dermatology, Ann & Robert H. Lurie Children's Hospital of Chicago. "There are several challenges associated with diagnosing and treating scabies, and what makes this therapeutic option unique is an entirely novel mechanism of action whereby spinosad – the active compound in Natroba – is delivered to the stratum corneum, the outermost layer of the epidermis, and targets mites at the site of infestation without penetrating deep into the dermis or entering systemic circulation. This product offers practitioners a new, proven option for the treatment of scabies."
About Natroba
Natroba (spinosad) Topical Suspension, 0.9% is an FDA-approved, targeted topical prescription therapy with multi-ectoparasitic indications. Natroba is indicated for the topical treatment of scabies infestations in adult and pediatric patients four (4) years of age and older and is the only FDA-approved, targeted topical prescription therapy that meets the latest FDA criteria for effectiveness and safety in treating scabies infestations.
Natroba is dosed as a single, full-body application. Click here for a video on how to properly apply Natroba.
About the Clinical Trials
All members of the household were treated with the same randomized treatment (Natroba or vehicle), whether or not the member of the household had an active scabies infestation. The patient applied a single application of Natroba or vehicle on Day 1 and returned for evaluation on Day 28. Efficacy was assessed as the proportion of index subjects who achieved complete cure 28 days after treatment. The studies showed that Natroba was not equivalent to vehicle in the percentage of index intent-to-treat subjects achieving complete cure at Day 28 (78.1% vs 39.6%, respectively; p-value <0.0001; n=206). Safety findings suggest the product was well-tolerated by patients as young as four years old.3,4
About Scabies & its Treatment
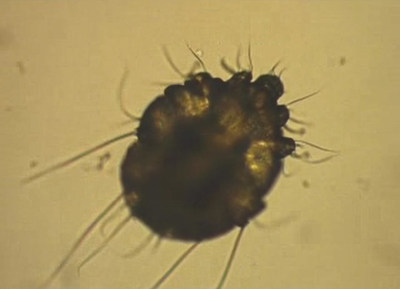
Scabies is a contagious skin disease caused by an infestation of the skin by the human itch mite Sarcoptes scabiei var. hominis. The microscopic scabies mite burrows into the upper layer of the skin where it lives and lays its eggs. The most common symptoms of scabies are intense itching and a pimple-like skin rash. The scabies mite is usually spread by direct, prolonged, skin-to-skin contact with a person who has scabies.9
Scabies affects people of all races and social classes and can spread rapidly under crowded conditions where there is frequent close body contact. There are an estimated 850,000 – 1 million cases of scabies in the United States each year.10
Permethrin 5% cream is offered as first-line topical therapy in the U.S. for the treatment of scabies, however in vitro studies and small investigator initiated in vivo trials have reported that efficacy appears to be decreasing which may be due to widespread resistance among other ectoparasites.11-14
"Several physicians familiar with Natroba in permethrin-resistant head lice geographies encouraged ParaPRO to look at scabies because they claimed to see similar resistance to 5% permethrin treatments," said Culpepper. "Natroba's FDA approval is not only a credit to the science behind the product, but also to the health care workers who share careful observations that inspire organizations like ParaPRO to pursue new cures."
INDICATION
Natroba Topical Suspension is a scabicide indicated for the topical treatment of scabies infestations in adult and pediatric patients four (4) years of age and older.
ADJUNCTIVE MEASURES
- Wash in hot water or dry-clean any bedding, clothing and towels used by anyone having scabies.
- If any member of a household presents with scabies, all household members should be treated with Natroba Topical Suspension.
IMPORTANT SAFETY INFORMATION
Natroba™ Topical Suspension contains benzyl alcohol and is not recommended for use in pediatric patients below the age of 4 years. The safety and effectiveness of Natroba™ Topical Suspension have not been established in pediatric patients less than 4 years of age with scabies infestation. Most common adverse events were: application site irritation (3%)* and dry skin (2%).
*Application site irritation also includes application site pain and burning sensation.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Please see full Prescribing Information for Natroba.
About ParaPRO
ParaPRO, LLC., a life-sciences organization dedicated to eradicating ectoparasites (bugs that live on the body's surface or just beneath), has developed a variety of diagnostic and educational tools to assist healthcare providers and parents in successfully identifying, managing and treating head lice infestations in adults and children. For more information, please visit ParaPRO.com/head-lice/.
References
- U.S. FDA (n.d.). Orange Book: Approved Drug products with Therapeutic Equivalence Evaluations. https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm. Accessed, 7/21/2021.
- IQVIA; April 5, 2021
- Natroba Prescribing Information
- Data on file, ParaPRO, LLC
- Summary of NDA approvals & receipts, 1938 to the present. US FDA website. https://www.fda.gov/about-fda/histories-product-regulation/summary-nda-approvals-receipts-1938-present. Accessed July 26, 2021.
- LaMattina J. Can the record-breaking number of FDA new drug approvals continue? Forbes website https://www.forbes.com/sites/johnlamattina/2019/01/09/can-the-record-breaking-number-of-fda-new-drug-approvals-continue/?sh=243f6ffcaa83 Published January 9, 2019. Accessed July 26, 2021
- Elimite (permethrin) Cream, 5% (NDA 19-855) FDA Approval Letter [Letter written August 25, 1989 to Burroughs Wellcome Company] (1989). U.S. Food and Drug Administration. Division of Anti-Infective Drug Products.
- ParaPRO, LLC & Concentrics Research (2018). A Phase 3, Randomized, Double Blind, Placebo-Controlled Study to Assess the Safety, Efficacy and Pharmacokinetics of Natroba™ (Spinosad) for the Treatment of Scabies (Clinical Study Protocol, Amd. 6.0, pp. 1-66).
- Centers for Disease Control and Prevention. Parasites. https://www.cdc.gov/parasites/scabies/. Accessed, 7/26/21.
- IQVIA; March 2019-March 2021.
- Cohen P R (March 25, 2020) Classic and Non-classic (Surrepticius) Scabies: Diagnostic and Treatment Considerations. Cureus 12(3): e7419. doi:10.7759/cureus.7419.
- Damian Meyersburg, Andreas Kaiser & Johann Wolfgang Bauer (2020) 'Loss of efficacy of topical 5% permethrin for treating scabies: an Austrian single-center study', Journal of Dermatological Treatment, DOI:10.1080/09546634.2020.1774489.
- Khalil S, Abbas O, Kibbi AG, Kurban M (2017) Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis 11(11): e0005920. https://doi.org/10.1371/journal.pntd.0005920.
- Mounsey KE et al. Scabies: Molecular perspectives and therapeutic implications in the face of emerging drug resistance Future Microbiol. 2008;3(1):57-66.
SOURCE ParaPRO, LLC



Share this article