
Philips receives U.S. FDA 510(k) clearance to market its solution for 'small parts' ultrasound imaging
Industry first, one transducer solution and tailored clinical tools for 'small parts' ultrasound imaging, combined with power of Philips EPIQ and Affiniti, assesses diseases and disorders in one of the fastest growing segments of ultrasound exams
AMSTERDAM and TAIPEI, Taiwan, Oct. 13, 2017 /PRNewswire/ -- Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market the new eL18-4 transducer with full solution for small parts imaging, which is used to assess diseases and disorders of small organs such as breasts, testicles and thyroid, as well as musculoskeletal injuries like sprains and tears. The Philips Ultimate Small Parts Solution features four key innovations that work together to help improve patient care: the eL18-4 PureWave linear array transducer, Philips MicroFlow Imaging, Philips Elastography and Philips Precision Biopsy. Philips will debut this latest solution, which is available on Philips EPIQ 7 and 5 and Affiniti 70 ultrasound systems, at the 16th World Federation for Ultrasound in Medicine and Biology (WFUMB) Congress in Taipei.
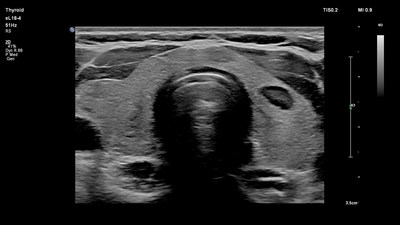
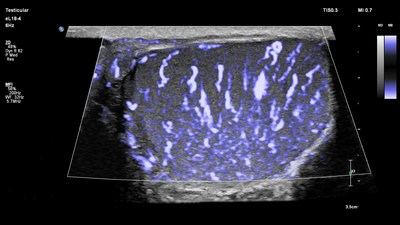
A vital tool for improving patient care, small parts imaging is a rapidly growing segment of ultrasound examinations for clinicians around the world. Its broad set of applications include assessment of diseases and disorders of small organs such as breasts, testicles and thyroid, as well as musculoskeletal injuries like sprains and tears. High-resolution ultrasound, such as that available on Philips EPIQ and Affiniti solutions, allows clinicians to visualize and characterize abnormalities and evaluate organ blood flow, guide treatment and perform biopsies of suspicious lesions.
"The superb image quality derived from Philips' breakthrough, ultra-broadband frequency transducer along with full solution Elastography support, helps clinicians make confident clinical decisions driven by an exam that is easier to perform," said Vitor Rocha, Ultrasound Business Leader at Philips. "With the new Philips Ultimate Small Parts Solution, clinicians now have the power to comprehensively assess and treat small parts and deliver better care for their patients with all-in-one functionality."
"My clinical experience with the new Philips eL18-4 small parts transducer has enabled excellent versatility and penetration that could easily replace my current transducers," said Dr. Lynwood Hammers, Hammers Healthcare Imaging, New Haven, CT. "I am able to see subtle lesions with great detail and excellent sensitivity for small vessel details. In my opinion, this new small parts transducer definitely elevates my clinical confidence and decreases indeterminate diagnosis."
New Capabilities Advance Confident Diagnoses
The Philips EPIQ Ultimate Small Parts Solution is comprised of a suite of features for small parts assessment. These include:
- The Philips eL18-4 transducer, allows fine-elevation focusing to deliver exceptional detail resolution and tissue uniformity for clinical solutions, including: thyroid, breast, testicular, musculoskeletal, vascular, bowel, pediatrics and obstetrics.
- Philips MicroFlow Imaging, provides remarkable sensitivity and detail for small vessel blood flow assessment.
- Complete Elastography solution with both strain and ElastQ Imaging shear wave capability, reveals definitive information on tissue stiffness.
- New precision biopsy capabilities, help minimize needle blind zones and support the ability to enhance the display of needle reflections – all to elevate confidence during interventional procedures.
Delivering Exceptional Care with Ultrasound
This eL18-4 small parts solution builds upon both the EPIQ and Affiniti platforms, enabling radiologists to have more confidence in their diagnosis, maximize productivity and connected workflow, and facilitate patient data security with best-in-class imaging solutions that fit clinicians' unique needs. Together, EPIQ and Affiniti deliver on Philips' commitment to improving patient care with industry-leading solutions.
Philips is a leader in cardiac ultrasound imaging, disease specific applications and services. The company is expanding its portfolio in OB/GYN, point-of-care and general imaging. For more information on Philips' general imaging ultrasound portfolio visit http://philips.to/2jPsYWq. Stop by the Philips booth E2 at WFUMB 2017, in Taipei, Taiwan, from October 13-17, to experience its latest advancements in general imaging ultrasound, and follow @PhilipsLiveFrom for updates throughout the congress.
For further information, please contact:
Alicia Cafardi
Philips Group Press Office
Mobile: +1 412 523 9616
Email: [email protected]
Twitter: @aliciacafardi
Sarah Haeger
Philips Ultrasound
Mobile: +1 206-920-8726
Email: [email protected]
Twitter: @sarahhaeger
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips' health technology portfolio generated 2016 sales of EUR 17.4 billion and employs approximately 71,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
SOURCE Royal Philips

Share this article