
--PYK-2101 Aims to Provide Dramatic Improvement in Patient Outcomes and Post-operative Experience Following Retinal Detachment Procedures--
CAMBRIDGE, Mass., Feb. 6, 2025 /PRNewswire/ -- Pykus Therapeutics, Inc., a clinical-stage medical technology company developing a novel retinal sealant, PYK-2101, to replace gas and oil during retinal detachment repair, today announced that it will present initial clinical data at the prestigious Vail Vitrectomy 2025 Meeting on February 10, 2025. The presentation will provide new insights into the development and clinical progress of PYK-2101, Pykus' lead investigational device designed to improve surgical outcomes and patients' post-operative experience compared to the current standard of care.
Presentation Details:
- Title: Use of Focal Retinal Sealants in Retinal Detachment Repair: Pilot Clinical Study Results
- Presenter: James A. Stefater, MD, PhD, President and Cofounder, Pykus Therapeutics
- Time: February 10, 2025, 6:30 am MST
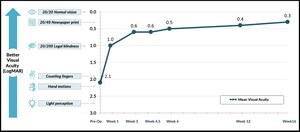
"More than 125,000 people undergo retinal reattachment procedures on a yearly basis in the US. Although our success rate is high, the current recovery process is uncomfortable and inconvenient for patients. In particular, the post-operative positioning requirements and limited vision associated with intraocular gas bubbles are significant limitations to a return to normal life. The Pykus solution would be a transformative advance," stated Carl Awh, MD, a retina specialist with Tennessee Retina, Nashville, TN.
PYK-2101 is a novel, patented biodegradable hydrogel developed to dramatically improve the surgical recovery experience by directly sealing retinal breaks without obscuring vision, and eliminates the need to position face-down. The sealant is intended to be safer and more effective during retina surgery compared to silicone oil or medical gases. This new approach aims to improve patient outcomes and patient experience after surgery.
About Vail Vitrectomy:
The Vail Vitrectomy Meeting was founded by Dr. Robert Machemer, the "father of modern retinal surgery," in 1975. The meeting, renowned for bringing together leading retina surgeons and researchers from all across the world, provides a platform to discuss advancements in vitreoretinal surgery and technology. The meeting is invitation-only and takes place once every three years.
About Pykus Therapeutics:
Pykus Therapeutics, Inc., based in Cambridge, MA, is a clinical-stage, medical technology company dedicated to advancing treatments for retinal and other ophthalmic diseases. Using technology originally developed by and licensed from the Mass. Eye and Ear Infirmary (now part of Mass General Brigham) at Harvard Medical School, the Company aims to deliver transformative solutions to improve surgical outcomes and enhance patient care. For more information, visit www.pykustherapeutics.com.
Contact:
Kelsey-Ann Leslie
Pykus Therapeutics
[email protected]
SOURCE Pykus Therapeutics







Share this article