
HIGHLIGHTS
- The first of the planned 24 participants in the Cohort Expansion Phase (Phase II) of the SECuRE trial has been treated with their first dose of 8 GBq of 67Cu-SAR-bisPSMA.This follows the recent recommendation by the Safety Review Committee (SRC) after the successful completion of the Dose Escalation Phase to commence enrollment for the Cohort Expansion Phase at the 8 GBq dose level, with an increase of the number of cycles from up to 4 to up to 6.
- This participant will be treated with the combination of 8 GBq of 67Cu-SAR-bisPSMA with enzalutamide (androgen receptor pathway inhibitor [ARPI]), as per the recent protocol amendment to include a subset of participants in the Cohort Expansion Phase to receive this combination.
- Prior to the start of the Cohort Expansion Phase, Clarity rolled out its improved 67Cu-SAR-bisPSMA product formulation. The enhanced formulation offers room temperature stability, supply and scalability, which are essential for late-stage clinical trials and streamlined commercial-scale manufacture.
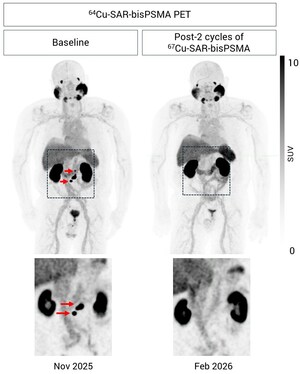
SYDNEY, April 15, 2025 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity" or "Company"), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for patients with cancer, is pleased to announce the treatment of the first participant with their first dose of 8 GBq of 67Cu-SAR-bisPSMA in the Cohort Expansion Phase of the SECuRE trial (NCT04868604)[1].
The dosing of this participant follows the recent successful completion of the Dose Escalation Phase (Phase I) of the trial and subsequent SRC recommendation to progress to the Cohort Expansion Phase (Phase II) at the 8 GBq dose level, with an increase in the total number of cycles from up to 4 to up to 6. This recommendation is based on the favourable safety profile and efficacy of 67Cu-SAR-bisPSMA observed to date[2].
This participant will be receiving the combination of 8 GBq of 67Cu-SAR-bisPSMA and enzalutamide (ARPI), allowed by a recent protocol amendment. This amendment incorporated an increase in the number of participants in this cohort from 14 to 24, in which a subset of participants will receive this combination therapy. These changes are aligned with the positive results from the Enza-p trial[3] and the ongoing discussions with and advice from key global medical experts in the field of prostate cancer, including the Company's Clinical Advisory Board members, Prof Louise Emmett and Prof Oliver Sartor, as well as the SRC.
The recently amended SECuRE trial protocol will also focus on the evaluation of metastatic castration-resistant prostate cancer (mCRPC) participants in the pre-chemotherapy setting. This aligns with Clarity's strategy of bringing 67Cu-SAR-bisPSMA to earlier stages of the disease and is based on its promising safety and efficacy data, especially in pre-chemotherapy participants treated in the SECuRE trial to date. In the Dose Escalation Phase, preliminary data showed that 92% of pre-chemotherapy participants (12/13) demonstrated prostate-specific antigen (PSA) drops greater than 35%, PSA reductions greater than 50% were reached in 61.5% (8/13) of participants, and reductions of 80% or more were achieved in 46.2% (6/13) of participants. These outstanding results were achieved despite many of the 13 pre-chemotherapy participants having considerable disease burden, being heavily pre-treated, and the majority of them only receiving a single dose of 67Cu-SAR-bisPSMA[2].
In preparation for the Cohort Expansion Phase, Clarity rolled out its improved 67Cu-SAR-bisPSMA product formulation. The enhanced formulation allows for room temperature stability, supply and scalability, which are essential for late-stage clinical trials and streamlined commercial-scale manufacture.
Clarity's Executive Chairperson, Dr Alan Taylor, commented, "We are incredibly excited about the progress we've achieved in the SECuRE trial to date and look forward to continuing to generate promising data in the Cohort Expansion Phase.
"With the latest protocol amendments ensuring that we are utilising the most recent advances and knowledge in the radiopharmaceutical space, we continue to be driven by the highest standards of clinical trial management and research. At Clarity, we are committed to working with various key opinion leaders in the field and incorporating the most recent findings into our study design to maximise the probability of clinical trial success and positive patient outcomes. As a result, we are focused on bringing 67Cu-SAR-bisPSMA to earlier lines of prostate cancer therapy, especially in the pre-chemotherapy setting, where we have seen very promising safety and efficacy to date. We are also investigating the benefits of combination therapy, where SECuRE participants are being treated with 67Cu-SAR-bisPSMA and enzalutamide based on the results from Prof Emmett's Enza-p trial and in consultation with global thought leaders in the prostate cancer space.
"We are committed to continuously improving our product and took the opportunity to advance our 67Cu-SAR-bisPSMA formulation prior to dosing our first patients in the Cohort Expansion Phase of the trial. The improvements also help us prepare for the large-scale manufacture in a potential Phase III trial and during commercialisation, allowing for room temperature stability with considerable advantages for supply and scalability. The ability to manufacture 67Cu-SAR-bisPSMA under room temperature reduces the likelihood of batch failures which lead to common supply issues and subsequent product shipment delays. Through the improvements in formulation, we hope that no patient is left waiting for their 67Cu-SAR-bisPSMA treatments.
"We thank our community, our team, Principal Investigators, members of the SRC, and especially the participants who have contributed to the SECuRE study so far. Armed with favourable safety and efficacy data from the Dose Escalation cohorts and with 3 US Food and Drug Administration (FDA) Fast Track Designations for the SAR-bisPSMA molecule, 1 for therapy and 2 for diagnostics, we are closer than ever to delivering on our ultimate goal of improving treatment outcomes for people with cancer."
About the SECuRE trial
The SECuRE trial (NCT04868604)[1] is a Phase I/IIa theranostic trial for identification and treatment of participants with PSMA-expressing mCRPC using 64Cu/67Cu-SAR-bisPSMA. 64Cu-SAR-bisPSMA is used to visualise PSMA-expressing lesions and select candidates for subsequent 67Cu-SAR-bisPSMA therapy. The trial is a multi-centre, single arm, dose escalation study with a cohort expansion involving approximately 54 participants in the US and Australia. The overall aim of the trial is to determine the safety and efficacy of 67Cu-SAR-bisPSMA for the treatment of prostate cancer.
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word "bis", which reflects a novel approach of connecting two PSMA-targeting agents to Clarity's proprietary sarcophagine (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a Targeted Copper Theranostic (TCT) that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
67Cu-SAR-bisPSMA and 64Cu-SAR-bisPSMA are unregistered products. The safety and efficacy of 67Cu-SAR-bisPSMA and 64Cu-SAR-bisPSMA have not been assessed by health authorities such as the US FDA or the Therapeutic Goods Administration (TGA). There is no guarantee that these products will become commercially available.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death in men worldwide[4]. Prostate cancer is the second-leading causes of cancer death in American men. The American Cancer Institute estimates in 2025 there will be about 313,780 new cases of prostate cancer in the US and around 35,770 deaths from the disease[5].
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious diseases. The Company is a leader in innovative radiopharmaceuticals, developing Targeted Copper Theranostics based on its SAR Technology Platform for the treatment of cancers in children and adults.
www.claritypharmaceuticals.com
For more information, please contact: |
|
Clarity Pharmaceuticals |
|
Dr Alan Taylor |
Catherine Strong |
Executive Chairperson |
Investor/Media Relations |
+61 406 759 268 |
References
- ClinicalTrials.gov Identifier: NCT04868604, https://clinicaltrials.gov/ct2/show/NCT04868604
- Clarity Pharmaceuticals. SECuRE trial update: 92% of pre-chemo participants experience greater than 35% drop in PSA levels across all cohorts. Cohort Expansion Phase commences. https://www.claritypharmaceuticals.com/news/secure-update/
- Emmett L et al. ENZA-p Trial Investigators; Australian and New Zealand Urogenital and Prostate Cancer Trials Group. Overall survival and quality of life with [177Lu]Lu-PSMA-617 plus enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer (ENZA-p): secondary outcomes from a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2025 Mar;26(3):291-299. doi: 10.1016/S1470-2045(25)00009-9.
- Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21834
- American Cancer Society: Key Statistics for Prostate Cancer. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
This announcement has been authorised for release by the Executive Chairperson.
SOURCE Clarity Pharmaceuticals







Share this article