
Second-generation delivery device offers cost-effective alternative to hospital care benefiting patients, providers and payors
Worsening heart failure is among the top three indications for hospitalizations in the elderly
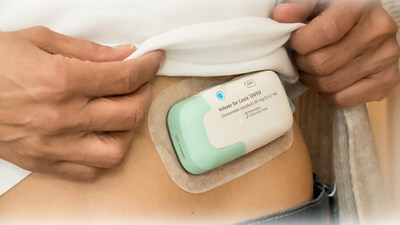
BURLINGTON, Mass., Oct. 8, 2025 /PRNewswire/ -- SQ Innovation, Inc. today announced that the U.S. Food and Drug Administration (FDA) has approved its drug-device combination Lasix® ONYU (furosemide injection) for the treatment of edema (due to fluid overload) in adult patients with chronic heart failure. Lasix ONYU was developed to enable subcutaneous infusion of furosemide outside the healthcare setting for selected patients, as prescribed by a clinician without the need for a healthcare professional to administer the drug.
About 6.7 million Americans suffer from heart failure, with the prevalence expected to rise to 8.7 million by 2030. Heart failure is a leading cause of hospitalizations for individuals aged 65 and older with approximately 1.2 million hospitalizations per year1.
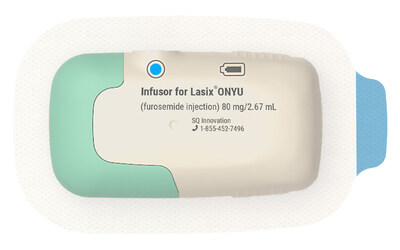
Lasix ONYU consists of a novel high-concentration formulation of furosemide combined with a state-of-the-art small Infusor for treatment at home. The innovative design includes a reusable unit that can be used for 48 treatments and a plastic sterile single-use unit that is discarded after treatment. The two-component design reduces manufacturing complexity and cost, allowing the product to be offered at a different, more favorable price point which is expected to reduce barriers to widespread adoption.
"Lasix ONYU has the potential to be transformative in the care of patients experiencing worsening heart failure due to fluid overload," said Pieter Muntendam, MD, founder, President and CEO of SQ Innovation. "Treating selected patients at home offers important benefits to patients, health systems and payors. We look forward to launching Lasix ONYU with leading health systems in the 4th quarter of 2025."
Bioavailability and diuretic response were determined in a clinical study in which Lasix ONYU demonstrated complete bioavailability (112%) resulting in similar diuresis (115%) and natriuresis (117%) when compared to the same dose given by IV bolus. The biphasic delivery of furosemide by the Infusor resulted in a tempered diuretic response while IV bolus administration led to a shorter period of more intense diuresis. The results of the study were published in a leading cardiovascular journal2.
"Heart failure is the most common serious medical condition in the U.S. and affects about one in four Americans during their lifetime. The number of patients affected is expected to double over the next 20 years and we currently already often lack adequate resources to take care of the 6.7 million patients affected presently – there are not enough beds, clinicians and funds", said Dr. Javed Butler, Professor of Medicine at University of Mississippi and President, Baylor Scott and White Research Institute. "The only two actionable solutions now are more widespread adoption of guideline directed medical therapy (GDMT) and treating more patients at home with products such as subcutaneous diuretics instead of hospitalization for intravenous diuretics."
"Decongestion through use of IV diuretics has been the cornerstone of treatment for reducing edema and hypervolemia in heart failure patients for over five decades," stated S. Craig Thomas, Immediate Past President of the American Association of Heart Failure Nurses (AAHFN), an organization dedicated to advancing nursing education, clinical practice, and research to improve outcomes for heart failure patients. "The availability of accessible, affordable, and novel options that do not require the presence of a healthcare professional allows for transformative new clinical care-delivery. This means patients who now would typically need to be hospitalized for several days of IV treatment can instead remain home, supported by periodic or remote monitoring. The significance of this shift away from inpatient care for patients, hospitals, and payers cannot be overstated."
Starting this quarter, Lasix ONYU will be available from leading pharmaceutical distributors enabling timely availability at participating medical facilities and affiliated retail pharmacies.
SQ Innovation is hosting a Conference Call and Webcast on Thursday October 9, at 4:30pm ET to introduce the product and answer questions from the community. Participating in the conference call will be:
- Pieter Muntendam, MD, President and CEO of SQ Innovation
- Mustafa M. Ahmed, MD, Professor of Medicine, Section Chief, Heart Failure, University of Florida Health, Gainesville, FL
- S. Craig Thomas, Nurse Practitioner, Advanced Heart Failure Center, University of Virginia Health System, Charlottesville, VA and Immediate Past President American Association of Heart Failure Nurses (AAHFN)
To register for the conference call go to this link Lasix ONYU Conference Call.
About Lasix ONYU
Lasix® ONYU is a drug-device combination that was approved by the U.S. Food and Drug Administration on October 7, 2025 for the treatment of edema in adult patients with chronic heart failure. The pharmaceutical component of Lasix ONYU is a novel, high-concentration formulation of the diuretic furosemide, at 30 mg/mL. It comes in a pre-filled glass cartridge containing 80 mg of furosemide in 2.67 mL. The Lasix ONYU Infusor consists of two main parts: the Reusable Unit and the Disposable Unit. The Reusable Unit is an electromechanical device that contains the battery, motor, and electronic components necessary for operation and safety functions. It can be used up to 48 times before it can be recycled. The Disposable Unit is a sterile, single-use plastic component that holds the drug cartridge. It includes a micropiston pump, fluid path, needle insertion and retraction mechanism, and a 29-gauge needle. After placement on the abdomen, the needle penetrates the skin when the device is activated. The Lasix ONYU Infusor slowly administers 80 mg furosemide over a period of five hours. This method results in significant diuresis similar to IV, but in a more controlled manner. This avoids the brief, intense diuretic effect that occurs with rapid IV infusion or injection. The advanced two component design offers benefits for patients, healthcare providers, payers, and the environment. For Important Safety Information, Prescribing Information and Instructions for Use, visit www.lasix-onyu.com.
INDICATION
Lasix® ONYU is a loop diuretic indicated for the treatment of edema in adult patients with chronic heart failure.
IMPORTANT SAFETY INFORMATION
Before using Lasix® ONYU, read the Instructions for Use and tell your healthcare provider about all your medical conditions, including if you are allergic to furosemide or any of the ingredients in Lasix ONYU, have trouble urinating, or if you are allergic to medical adhesives.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines.
Warning: Only use the Lasix ONYU Prefilled Cartridge with the Lasix ONYU Infusor. Do not use insulin cartridges or other medicine cartridges in the Lasix ONYU Infusor. Doing so could cause severe injury.
What are the possible side effects of Lasix ONYU?
Dehydration: Lasix ONYU is a diuretic that can make you lose a lot of fluid and with it electrolytes. You may get a dry mouth, have increased thirst, get muscle pains or cramps, have reduced urine output or your urine may be more yellow than normal, you may get a headache, get dry skin, or have nausea or vomiting. Your healthcare provider may check your electrolytes while receiving Lasix ONYU.
Low Blood Pressure: Lasix ONYU may lower your blood pressure temporarily. You may feel lightheaded or dizzy. This usually happens when you stand. Getting up slowly may help.
High Blood Sugar: Lasix ONYU may increase blood sugar (glucose) levels if you have diabetes mellitus.
Loss of Hearing: Lasix ONYU can cause ringing in your ears. If so tell your healthcare provider.
Risk of Sunburn: Your skin may be more sensitive to sunlight while taking Lasix ONYU.
Infusion Site Reactions: Lasix ONYU can cause infusion site pain, bruising and temporary swelling or redness at the site of the Infusor.
Incomplete Dosing: Make sure the Infusor does not get wet during use. Also limit your physical activities. Some movements or when it gets wet may stop the infusion and you may not get all the medication.
These are not all the possible side effects of Lasix ONYU. For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
Please see the full Prescribing Information and Instructions for Use .
About SQ Innovation
SQ Innovation, Inc. is a privately held Swiss biopharmaceutical company with offices in Zug, Switzerland, Burlington, MA, USA, and Rotterdam, The Netherlands. The company was founded to develop and commercialize innovative, cost-effective therapies for subcutaneous delivery, enabling at-home treatment for conditions that are usually managed during hospitalizations. SQ Innovation has developed a novel drug-device combination for treating edema in adult patients with chronic heart failure — a condition typically requiring intravenous administration of diuretics in a hospital setting. Lasix ONYU was developed with consideration for patients, payors, healthcare providers, and environmental impact. Lasix ONYU received approval from the US Food and Drug Administration on October 7, 2025. For more information about Lasix ONYU, including important safety information and the full prescribing information, please visit www.Lasix-ONYU.com.
For Media Inquiries:
[email protected]
1 Bozkurt B, Ahmad T, Alexander K, Baker WL, Bosak K, Breathett K, Carter S, Drazner MH, Dunlay SM, Fonarow GC, Greene SJ, Heidenreich P, Ho JE, Hsich E, Ibrahim NE, Jones LM, Khan SS, Khazanie P, Koelling T, Lee CS, Morris AA, Page RL 2nd, Pandey A, Piano MR, Sandhu AT, Stehlik J, Stevenson LW, Teerlink J, Vest AR, Yancy C, Ziaeian B; WRITING COMMITTEE MEMBERS. HF STATS 2024: Heart Failure Epidemiology and Outcomes Statistics An Updated 2024 Report from the Heart Failure Society of America. J Card Fail. 2024 Sep 14:S1071-9164(24)00232-X. doi: 10.1016/j.cardfail.2024.07.001. Epub ahead of print. PMID: 39322534.
2 Joanna Osmanska, Katriona Brooksbank, Kieran F Docherty, Stacy Robertson, Kirsty Wetherall, Alex McConnachie, Jerry Hu, Roy S Gardner, Andrew L Clark, Iain B Squire, Paul R Kalra, Pardeep S Jhund, Pieter Muntendam, John J V McMurray, Mark C Petrie, Ross T Campbell, A novel, small-volume subcutaneous furosemide formulation delivered by an abdominal patch infusor device in patients with heart failure: results of two phase I studies, European Heart Journal - Cardiovascular Pharmacotherapy, 2023;, pvad073, https://doi.org/10.1093/ehjcvp/pvad073
SOURCE SQ Innovation








Share this article