
ZEISS expands DORC portfolio and further strengthens digital footprint with improved workflow efficiency and digital visualization
Showcasing at AAO 2025:
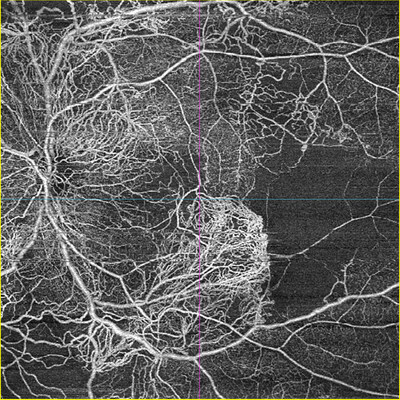
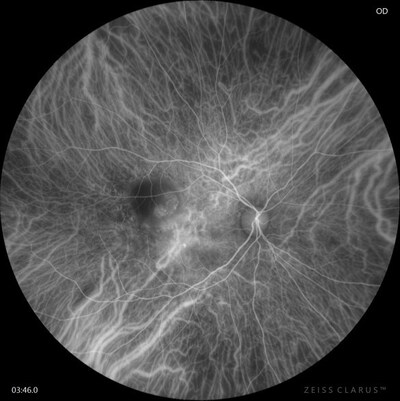
- NEW 510k CLEARANCE SUPPORTS MORE INFORMED DECISION MAKING & PATHOLOGY DETECTION: Showcasing optical coherence tomography angiography (OCT-A) with ZEISS CIRRUS AngioPlex® with noninvasive imaging of the retinal and choroidal vasculature; now with 510k clearance and CE approval, ICGA for CLARUS® 700 offers FA + ICG simultaneous capture.
- OPTIMIZED RETINAL SURGICAL EXPERIENCE: Announcing updates to DORC instruments to expand surgeons' options, including new gauges added to the range of subretinal injection cannulae and a new Midfield endoillumination light probe with directed light; updated CALLISTO eye® software on the ZEISS ARTEVO 850 and ZEISS ARTEVO 750 supports increased visualization.
- IMPROVED GLAUCOMA MANAGEMENT FOR CATARACT PATIENTS: Demonstrating a fully integrated diagnostic and treatment solution for managing comorbid cataract/glaucoma patients to support more efficient and confident decision making.
- ENHANCED OPHTHALMIC RESEARCH AND COLLABORATION: Featuring the AI-ready ZEISS Research Data Platform (RDP), which can empower retina specialists to transform their clinical hypotheses into measurable outcomes; previewing the next evolution of browser-based digital data management which aggregates clinical data at scale, powers advanced analytics, and enables seamless collaboration across eye care professionals for more personalized patient care delivery.
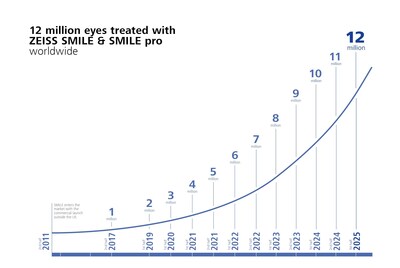
- REFRACTIVE INDUSTRY MILESTONE: Celebrating more than 12 million eyes treated with ZEISS SMILE and ZEISS SMILE pro.
JENA, Germany and DUBLIN, Calif., Oct. 7, 2025 /PRNewswire/ -- ZEISS Medical Technology will showcase its latest enhancements in ophthalmic visualization and digital workflow innovation at the American Academy of Ophthalmology (AAO) conference from Oct. 18 - 20, 2025, in Orlando, FL.
"ZEISS continues to be at the forefront of optical and digital innovation, advancing our clinical workflows to be more efficient and empowering for doctors and surgeons around the world to deliver more precise and personalized care for their patients," says Magnus Reibenspiess, Head of Strategic Business Unit Ophthalmology at ZEISS Medical Technology.
"We're delivering important enhancements to our expanding portfolio across ZEISS and DORC solutions to meet the growing needs of ophthalmologists worldwide," said PT Cheong, Head of Global Sales for Ophthalmology, ZEISS Medical Technology. "From diagnostics to surgery and post-op management, our advanced technology solutions support more confident, personalized patient care."
Advancing visualization and efficiency throughout the spectrum of retinal care
The ZEISS Retina Workflow offers a digitally connected solution from diagnostics through surgery and post-op care, supporting healthcare professionals in advancing retinal care with enhanced data continuity, procedural efficiency, and clinical decision-making across the retina care pathway.
At AAO, ZEISS will showcase multiple diagnostic enhancements supporting improved decision making and pathology detection through gold-standard imaging technology. Now with 510k clearance and CE approval1, the CLARUS® 700 with ICGA provides high-resolution early-phase to late-phase ultra-widefield imaging. New features include standalone ICG, simultaneous FA-ICG capture, and an added movie mode for all angiography modalities: standalone FA, standalone ICG, and simultaneous FA-ICG. These advancements underscore ZEISS's commitment to delivering cutting-edge solutions that enhance clinical outcomes and streamline the diagnostic process for healthcare professionals.
Additionally, this year marks the 100-year anniversary of advancements in ZEISS fundus imaging. In 1926, ZEISS and Johan Nordenson released the first commercially available fundus camera, laying the foundation for a century of innovation in ocular imaging technology.
ZEISS will also showcase its latest in OCT technology, providing enhanced details and insights to assist eyecare practitioners when examining patients and managing diseases. The implementation of best practices is helpful to maximize the clinical utility of OCT-A. In recognition of this, ZEISS has released OCT-A Simplified, a step-by-step eBook by Ricardo Luz Leitão Guerra, MD, MSc, FICO. The guidebook makes OCT-A more accessible to eyecare practitioners, covering image acquisition, analysis, and interpretation providing practical insights clinicians can apply immediately in clinical decision-making. Copies of the OCT-A Simplified ebook will be available at AAO at the ZEISS booth #2261.
ZEISS continues to offer the latest in OCT solutions, most recently gaining CE mark for CIRRUS PathFinder, an innovative clinical support tool with artificial intelligence (AI) fully integrated to enable more confident decision-making and accelerate a clinician's patient care workflow with OCT interpretation assistance, supporting improved surgical planning, particularly for high-volume clinics.
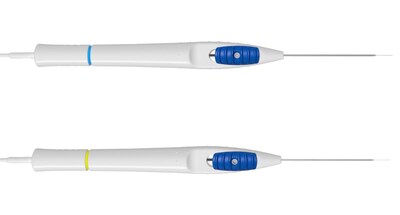
To further optimize the surgical experience for vitreoretinal surgeons, ZEISS will showcase new updates2 to its DORC instrument portfolio within the ZEISS Retinal Workflow.
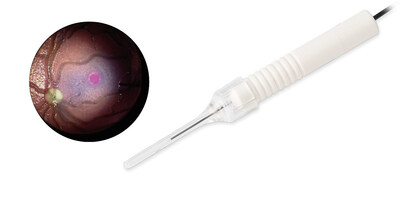
- New gauges added to the company's range of subretinal injection cannulae: 25G and 27G. 3 The subretinal cannulas are designed for injection of fluids and/or gases into subretinal space and feature a 41G extendible tip for a smooth and uninterrupted sliding motion. The range is compatible with EVA NEXUS's INICIO micro-injection system. EVA NEXUS is the only system cleared by the FDA for subretinal injection in the U.S.
- The new Midfield endoillumination light probe4 with directed light. The light probe provides a homogeneous field of illumination of about 60° with sharply defined edges, enabling surgeons to effectively distinguish between different media, such as vitreous, BSS and air. The new light probe is also suitable for trans-scleral illumination and comes with a trans-scleral depressor as standard. The probe is available in three gauges: 23G, 25G and 27G.5
- The DORC EVA NEXUS™ platform, uniquely suited to address both retina and cataract surgery, is now available with angled EquiPhaco designed for optimal irrigation flow. EquiPhaco, combined with EVA NEXUS SMART IOP™, allows for a more stable, lower anterior chamber pressure during procedures.
Lastly, ZEISS will showcase its updated CALLISTO eye® software for ZEISS ARTEVO 750 and ZEISS ARTEVO 850, allowing for visualization of retinal structures on the 3D screen of the ZEISS ARTEVO 850 with up to 30% higher magnification6. Advanced camera settings, including automatic exposure control, provide customization of the visualization experience to a surgeon's individual preferences. In addition, the software update now also offers reference image matching for toric IOL implantations in either the eyepiece of the ZEISS ARTEVO 750 or the 3D monitor of the ZEISS ARTEVO 850 as well as optional 3D recording on the ZEISS ARTEVO 850.
Improved efficiency for surgery planning through integrated glaucoma support
ZEISS diagnostic and digital solutions integrated into surgical ophthalmic workflows can help surgeons visualize more, connect insights across a workflow, and make data-driven decisions, including earlier detection of eye conditions that may affect surgical planning and outcomes. At AAO, ZEISS will demonstrate a fully integrated solution connecting cataract and glaucoma workflows to support more efficient and confident decision making.
Glaucoma is the most common comorbidity in cataract surgery. According to a recent study7, around 20% of patients who undergo cataract procedures also have glaucoma or ocular hypertension. ZEISS offers integrated diagnostic and therapeutic solutions to support earlier detection and management of glaucoma in cataract patients, helping to improve long-term outcomes of cataract surgery. Devices including the ZEISS SL 800, CLARUS®, CIRRUS® OCT, ARTEVO® 750, and VISULAS® are shared across the cataract and glaucoma workflows and seamlessly connected via FORUM® for complete data continuity.
Empowering the future of ophthalmology with enhanced research and collaboration tools
At AAO, the company will feature the ZEISS Research Data Platform (RDP), a secure, cloud-based, AI-driven solution designed for multi-center research and real-world clinical evidence, enabling retina specialists to translate their clinical hypotheses into measurable outcomes. With built-in support for AI algorithm training and biomarker generation, the ZEISS RDP allows researchers to harness complex data and drive innovation without compromising on compliance or collaboration.
ZEISS will also preview the next evolution of browser-based digital data management solution that seamlessly integrates with EMR systems to aggregate essential medical data and can connect diagnostic data from ZEISS and non-ZEISS devices. The solution provides actionable clinical insights through color-coded alerts, trend analysis for OCT and Visual Field data, and interactive analyses. Offered in flexible subscription packages, providers can select the configuration that fits their practice needs.
Celebrating continued excellence in refractive innovation
ZEISS continues to extend its global, refractive momentum with the celebration of more than 12 million eyes treated withSMILE® and SMILE® pro, reflecting the growing international adoption of safe and effective lenticule extraction solutions. ZEISS SMILE and ZEISS SMILE pro continue to be leading solutions trusted by surgeons for the technology's proven reliability and effective treatment with the VisuMax® and VISUMAX® 800 from ZEISS.
ZEISS will showcase its latest offerings and new innovations at the American Academy of Ophthalmology (AAO) conference from Oct. 18 - 20, 2025, in Orlando, FL, at booth #2261.
For more information, visit www.zeiss.com/med.
1Not available for commercial sale in specific markets until January 2026. For availability inquiries, please contact your ZEISS sales representative.
2Products are cleared for sales in the U.S. only.
3Product References: 1270.EXT25: 25G Extendible subretinal injection cannula (41G); 1270.EXT27: 27G Extendible subretinal injection cannula (41G).
4Product is manufactured by Peregrine Surgical LLC, a DORC/ZEISS company and is cleared for sales in the U.S. only.
5Product References: 3269.M06 23G Midfield Endoillumination Probe, including scleral depressor, 3269.M05 25G Midfield Endoillumination Probe, including scleral depressor, 3269.M04 27G Midfield Endoillumination Probe, including scleral depressor.
6Compared to previous CALLISTO eye 5.0 software version.
7Skalicky, S.E., Martin, K.R., Fenwick, E., Crowston, J.G., Goldberg, I. and McCluskey, P. (2015), Cataract and quality of life in glaucoma. Clin Experiment Ophthalmol, 43: 335-341. https://doi.org/10.1111/ceo.12454.
Not all products, services or offers are approved or offered in every market and approved labeling and instructions may vary from one country to another. For country-specific product information, see the appropriate country website. Product specifications are subject to change in design and scope of delivery as a result of ongoing technical development. The statements of the healthcare professionals reflect only their personal opinions and experiences and do not necessarily reflect the opinion of any institution that they are affiliated with. The healthcare professionals alone are responsible for the content of their experience reported and any potential resulting infringements. Carl Zeiss Meditec AG and its affiliates to not have clinical evidence supporting the opinions and statements of the health care professionals nor accept any responsibility or liability of the healthcare professionals' content. The healthcare professionals have a contractual or other financial relationship with Carl Zeiss Meditec AG and its affiliates and have received financial support.
Contact for investors
Sebastian Frericks
Head of Group Finance & Investor Relations
Carl Zeiss Meditec AG
Phone: +49 3641 220 116
Mail: [email protected]
Contact for the press
Frank Smith
Head of Global Communications
Ophthalmology, Carl Zeiss Meditec Inc.
Phone: +49 3641 220 331
Mail: [email protected]
Brief Profile
Carl Zeiss Meditec AG (ISIN: DE0005313704), which is listed on the MDAX and TecDAX of the German stock exchange, is one of the world's leading medical technology companies. The Company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. The Company offers complete solutions, including implants and consumables, to diagnose and treat eye diseases. The Company creates innovative visualization solutions in the field of microsurgery. With 5,730 employees worldwide, the Group generated revenue of €2,066.1m in fiscal year 2023/24 (to 30 September).
The Group's head office is located in Jena, Germany, and it has subsidiaries in Germany and abroad; more than 50 percent of its employees are based in the USA, Japan, Spain and France. The Center for Application and Research (CARIn) in Bangalore, India and the Carl Zeiss Innovations Center for Research and Development in Shanghai, China, strengthen the Company's presence in these rapidly developing economies. Around 39 percent of Carl Zeiss Meditec AG's shares are in free float. Approx. 59 percent are held by Carl Zeiss AG, one of the world's leading groups in the optical and optoelectronic industries.
For further information visit: www.zeiss.com/med
SOURCE Carl Zeiss Meditec AG







Share this article