
Ligand and SQ Innovation Enter into Exclusive Worldwide Captisol® License and Supply Agreements for High-Concentration Furosemide Formulation
SQ Innovation will use Ligand's Captisol technology to achieve a smaller delivery volume for more cost-effective diuretic treatment in heart failure.
The smaller delivery volume enables use of established drug container and device technology developed for insulin and other biologics, resulting in faster development and reduced cost.
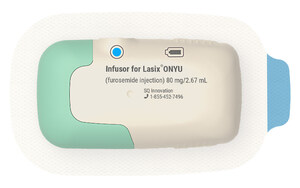
SQ Innovation to use advanced 3 mL drug delivery platform by Sensile Medical AG, a Gerresheimer company, for the novel treatment.
SAN DIEGO and ZUG, Switzerland, July 8, 2019 /PRNewswire/ -- Ligand Pharmaceuticals Incorporated (NASDAQ: LGND) and SQ Innovation AG today announced that the two companies have entered into long-term, exclusive commercial license and supply agreements for use of Ligand's Captisol® technology in the formulation of high-concentration furosemide for the treatment of edema in patients with heart failure. SQ Innovation is developing a novel drug-device combination for cost-effective subcutaneous delivery of diuretics that currently require intravenous administration by a certified healthcare professional. Ligand is eligible to receive potential milestone payments and royalties, as well as revenue from materials sales of Captisol.
"Solubility challenges, such as those faced by furosemide, are precisely what our Captisol technology is designed to address," said John Higgins, CEO of Ligand. "We are delighted to add SQ Innovation to our growing list of partner companies for our technology platforms, with more than 200 programs funded by partners, or Shots on Goal."
"A heart failure patient should not have to be in hospital to receive effective diuretic therapy for fluid overload," said Bertram Pitt MD, professor of medicine emeritus at the University of Michigan School of Medicine, Ann Arbor, MI. "A treatment option that provides IV strength diuresis without the need for venous access would enable the development of novel strategies, which could reduce hospitalizations for HF with a resultant improvement in quality of life and a reduction in health care costs."
"The novel Captisol-enabled furosemide formulation allows us to take advantage of the mature and cost-effective drug-delivery products of the diabetes/insulin space," said Pieter Muntendam MD, CEO of SQ Innovation AG. "Once approved, this new furosemide option may reduce the economic and patient burden of heart failure in the U.S. and Europe."
About Fluid Overload in Heart Failure
Heart failure is a common, complex and serious condition affecting approximately 6.2 million people in the U.S. and 26 million people worldwide. Many patients with heart failure experience episodes of worsening symptoms due to fluid overload. Current therapy options for these episodes include an increase in oral medication or, when oral treatment is not sufficiently effective, intravenous treatment, typically delivered in an emergency room or hospital. Subcutaneous infusion by means of a mini pump adhered to the skin may offer a solution for patients who otherwise do not require hospital care.
Fluid overload in heart failure is responsible for approximately $14 billion in Medicare spending, or approximately 3.9% of the Medicare budget, making it one of the most expensive therapies for the elderly. The high spending is attributable to in-patient care for diuretic treatment which accounts for approximately 7% of Medicare hospital admissions. In addition, a hospital stay is associated with risks of adverse outcomes due to rapid muscle loss and functional decline experienced by many elderly patients during their hospital stay and recovery.
About Captisol-enabled Furosemide
Furosemide is the leading diuretic used in patients with heart failure. It is administered as a daily oral therapy in most patients with heart failure, and intravenously in the hospital when oral medicine is insufficient to reduce fluid overload. Furosemide is poorly soluble, particularly at the neutral pH level required for subcutaneous delivery. Until now furosemide products suitable for human use have a low concentration and require 8-10mL to deliver the common therapeutic dose of 80mg. Although delivery of 8-10mL of fluids is possible in principle, there is currently no FDA-approved product in a drug cartridge or delivered by a patch pump with a volume greater than 3.5mL.
SQ Innovation's objective is to develop cost-effective therapies for the treatment of edema and other conditions where and when needed for patients who otherwise do not need to be in a hospital or emergency room. Instead of developing a drug container and device that accommodates a low-concentration drug, SQ Innovation conducted formulation research that led to the filing of multiple patent applications in 2018 and 2019. The broad formulation research program included a range of potential methods to improve the solubility of loop diuretics at neutral pH. This research revealed that beta-cyclodextrins, particularly the proprietary beta-cyclodextrin Captisol, enabled a high concentration of furosemide at neutral pH. This allowed 80mg of furosemide to be delivered using less than 3mL of drug formulation. SQ Innovation owns patent applications related to the use of cyclodextrins with loop diuretics that, when issued, would provide patent protection through 2039. Additionally, this formulation is subject to patents issued to Ligand subsidiary CyDex Pharmaceuticals regarding its Captisol product and technology that provide patent protection through 2034.
About SQ Innovation AG
SQ Innovation is a privately-held Swiss biopharmaceutical company located in Zug, Switzerland, and Burlington, MA, that was established to develop and commercialize innovative cost-effective therapies for subcutaneous delivery of pharmaceutical products where and when needed. Its first product, for the treatment of fluid overload (edema) in patients with heart failure, is currently under development following a plan that has been reviewed with the US-FDA. The novel combination product is based on the Sensile Medical micro-pump technology and its small-volume cartridge-based platform. The device design uses a reusable motorized component and disposable cartridge, which minimizes waste and creates cost-effective treatment options in numerous therapy areas, including treatment of edema in heart failure. The products are subject to regulatory review and approval. For more information visit www.furosemide.com.
About Captisol®
Captisol is a patent-protected, chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs. Captisol was invented and initially developed by scientists in the laboratories of Dr. Valentino Stella, University Distinguished Professor at the University of Kansas' Higuchi Biosciences Center, for specific use in drug development and formulation. This unique technology has enabled several FDA-approved products, including Amgen's Kyprolis®, Baxter International's Nexterone®, Acrotech Biopharma's EVOMELA®, Melinta Therapeutics' Baxdela™, Sage Therapeutics' ZULRESSO™, Merck & Co.'s Noxafil™ and Pfizer's Vfend™. There are many Captisol-enabled products currently in various stages of development.
About Ligand Pharmaceuticals
Ligand is a biopharmaceutical company focused on developing or acquiring technologies that help pharmaceutical companies discover and develop medicines. Our business model creates value for stockholders by providing a diversified portfolio of biotech and pharmaceutical product revenue streams that are supported by an efficient and low corporate cost structure. Our goal is to offer investors an opportunity to participate in the promise of the biotech industry in a profitable, diversified and lower-risk business than a typical biotech company. Our business model is based on doing what we do best: drug discovery, early-stage drug development, product reformulation and partnering. We partner with other pharmaceutical companies to leverage what they do best (late-stage development, regulatory management and commercialization) to ultimately generate our revenue. Ligand's Captisol platform technology is a patent-protected, chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs. OmniAb® is a patent-protected transgenic animal platform used in the discovery of fully human mono- and bispecific therapeutic antibodies. The Vernalis Design Platform (VDP) integrates protein structure determination and engineering, fragment screening and molecular modelling with medicinal chemistry, to enable success in novel drug discovery programs against highly-challenging targets. Ligand has established multiple alliances, licenses and other business relationships with the world's leading pharmaceutical companies including Amgen, Merck, Pfizer, Gilead, Janssen, Baxter International and Eli Lilly. Follow Ligand on Twitter @Ligand_LGND.
Forward-Looking Statements
This press release contains forward-looking statements by Ligand that involve risks and uncertainties and reflect Ligand's judgment as of the date of this report. These forward-looking statements include comments regarding the development, usefulness and commercial viability of Captisol-enabled furosemide. Actual events or results may differ from Ligand's expectations. For example, SQ Innovation might not execute its development and commercialization efforts effectively, regulatory approval of SQ Innovation's Captisol-enabled furosemide product may be delayed or withheld, or approved reimbursement levels for SQ Innovation's Captisol-enabled furosemide product might not be high enough to enable successful commercial introduction. Many of these risks also apply to the other programs which comprise Ligand's shots-on-goal portfolio. The failure to meet expectations with respect to any of the foregoing matters may reduce Ligand's stock price. Additional information concerning these and other important risk factors affecting Ligand (including Ligand's current reliance on revenues based on sales of Kyprolis®, and various risks to which Ligand's Captisol cyclodextrin operations are subject) can be found in Ligand's prior press releases and its periodic filings with the Securities and Exchange Commission (including its Form 10-K filed on February 28, 2019), available at www.sec.gov, as updated by subsequent periodic reports filed with the Securities and Exchange Commission. Ligand disclaims any intent or obligation to update these forward-looking statements beyond the date of this report. This caution is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.
SOURCE SQ Innovation







Share this article