
PARIS, July 31, 2017 /PRNewswire/ -- Sanofi (NYSE: SNY; EURONEXT: SAN)
| Q2 2017 |
Change |
Change |
Change |
H1 2017 |
Change |
Change |
Change |
|
| Business net income(1) |
€1,696m |
+1.0% |
-0.5% |
- |
€3,491m |
+2.6% |
+0.3% |
- |
| Business EPS(1) |
€1.35 |
+3.1% |
+1.5% |
- |
€2.77 |
+4.9% |
+2.7% |
- |
Second-quarter and first-half 2017 accounts reflect the acquisition of the former Boehringer Ingelheim Consumer Healthcare (CHC) business and the disposal of the Animal Health business (completed on January 1, 2017(3)). In accordance with IFRS 5 (Non-Current Assets Held for Sale and Discontinued Operations), Animal Health results in 2016 and gain on disposal in 2017 are reported separately. Second-quarter and first-half 2017 income statements also reflect the consolidation of European operations related to Sanofi vaccine portfolio, following the termination of the Sanofi Pasteur MSD joint venture (SPMSD JV) with Merck at the end of December 2016.
(1) In order to facilitate an understanding of operational performance, Sanofi comments on the business net income statement. Business net income is a non-GAAP financial measure (see Appendix 10 for definitions). The consolidated income statement for Q2 2017 and H1 2017 is provided in Appendix 3 and a reconciliation of IFRS net income reported to business net income is set forth in Appendix 4; (2) CS: constant structure: adjusted for BI CHC business, termination of SPMSD and others; (3) The closing of the disposal of Merial in Mexico is expected in 2017
Sanofi Chief Executive Officer, Olivier Brandicourt, commented:
"Sanofi Genzyme, Sanofi Pasteur and Emerging Markets were once again major contributors to our performance in the quarter. The continued growth of these businesses, together with disciplined expense management, enabled us to more than offset the headwinds in our Diabetes franchise. Consequently, we feel confident in our full-year outlook and raise our 2017 business EPS guidance. We are also encouraged by the strong uptake of Dupixent® in the U.S. and the approval of Kevzara®. The initiation of Phase 3 studies in additional indications for dupilumab, the Phase 2/3 programs with the anti PD-1 in multiple cancer indications and fitusiran in hemophilia were significant R&D milestones in the second quarter."
Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8093352-sanofi-earnings-results-q2-2017/
Q2 2017 sales growth supported by Specialty Care, Vaccines and Emerging Markets
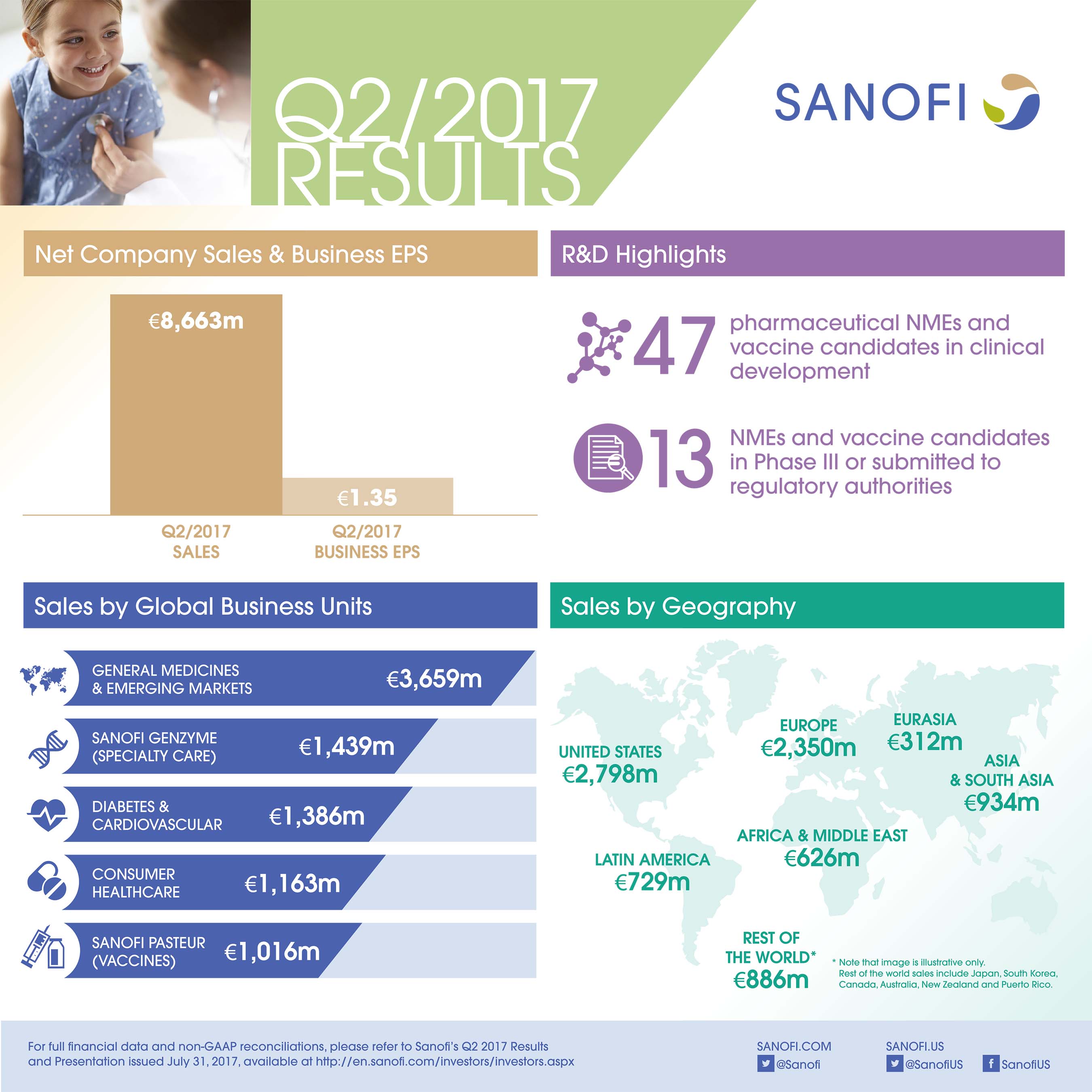
- Net sales were €8,663 million, up 6.4% on a reported basis and 5.5% at CER reflecting the change in scope of the CHC and vaccines Global Business Units (GBUs). At CER and CS, net sales were up 0.6%.
- Sanofi Genzyme GBU grew 14.3% at CER driven primarily by continued strong sales growth in Multiple Sclerosis; strong U.S. launch of Dupixent® in atopic dermatitis driven by high unmet medical need and early market access.
- Sanofi Pasteur GBU grew 19.2% at CER and CS as a result of strong sales of pediatric combinations and Menactra®.
- Diabetes and Cardiovascular GBU sales were down 15.0% at CER; Global Diabetes franchise sales decreased 12.2%.
- CHC GBU sales were stable at CER and CS mainly due to seasonality in Europe.
- Emerging Markets sales increased 6.6% at CER and CS driven by robust contribution from China.
2017 business EPS guidance at CER raised on first-half financial results and disciplined expense management
- Q2 2017 business operating income of €2,299 million, up 4.1% at CER and constant structure.
- Q2 2017 business EPS grew 1.5% at CER to €1.35 and increased 3.1% on a reported basis.
- Sanofi now expects 2017 business EPS to be broadly stable at CER, barring unforeseen major adverse events.
- Currency impact on 2017 business EPS is estimated to be approximately +1% at the average June 2017 exchange rates.
Sustaining innovation in R&D
- Positive CHMP opinion received for Dupixent® in the EU.
- Kevzara®, an anti IL6 for the treatment of rheumatoid arthritis, approved in the EU in June.
- Initiation of Phase 3 ATLAS program for fitusiran in patients with hemophilia.
- Phase 2/3 studies started for SAR439684 (anti PD-1) in Non-Small Cell Lung Cancer and Basal Cell Carcinoma.
R&D update
Regulatory update
Regulatory updates since the publication of first-quarter results on April 28, 2017 include the following:
- The European Commission granted marketing authorization for Insulin lispro Sanofi® (100 Units/mL) in July for the treatment of diabetes in adults and children. This followed the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) positive opinion in May.
- In July, the European Medicines Agency's CHMP adopted a positive opinion recommending the granting of the marketing authorization of Dupixent® (dupilumab) for use in adults with moderate-to-severe atopic dermatitis who are candidates for systemic therapy.
- In June, the European Commission granted marketing authorization for Kevzara® (sarilumab) in combination with methotrexate for the treatment of moderately to severely active rheumatoid arthritis (RA) in adult patients. In May, the U.S. Food and Drug Administration (FDA) also approved Kevzara® for the treatment of adult patients with moderately to severely active RA.
At the end of July 2017, the R&D pipeline contained 47 pharmaceutical new molecular entities (excluding Life Cycle Management) and vaccine candidates in clinical development of which 13 are in Phase 3 or have been submitted to the regulatory authorities for approval.
Portfolio update
Phase 3:
- In June, positive results from two Phase 3b/4 ODYSSEY-DM trials in patients with diabetes were announced. In the studies, Praluent® (alirocumab), when administered on top of maximally tolerated doses of statins, significantly reduced low-density lipoprotein cholesterol (LDL-C), the primary endpoint of the ODYSSEY DM-INSULIN study, and was superior to usual care in reducing non-high-density lipoprotein cholesterol (non-HDL-C), the primary endpoint of the ODYSSEY DM-DYSLIPIDEMIA study.
- In June, a Phase 3 study evaluating dupilumab in persistent asthma despite the use of medium to high dose of Inhaled Corticosteroid and a LABA (Long-Acting Beta Agonist) was initiated in 6-11 years population. Another Phase 3 evaluating dupilumab in moderate-to-severe atopic dermatitis in 12-17 years population was initiated in April.
- In May, a Phase 3 study evaluating SAR439684, a PD-1 inhibitor, in 1st line Non-Small Cell Lung Cancer started.
Phase 2:
- In July, Sanofi and Alnylam announced new positive results from the ongoing Phase 2 open-label extension (OLE) study with fitusiran in patients with hemophilia A and B, with or without inhibitors. These results were presented at the International Society on Thrombosis and Haemostasis (ISTH) 2017 Congress. The updated clinical results of this study showed that the safety and tolerability profile of fitusiran remains encouraging, with no thromboembolic events. Based on these results, the companies initiated the Phase 3 ATLAS program for fitusiran in patients with hemophilia A and B with or without inhibitors.
- In July, a Phase 2 study evaluating SAR439684, a PD-1 inhibitor, in advanced Basal Cell Carcinoma started.
Phase 1:
- SAR439459 (anti-TGF-β) entered into Phase 1 in monotherapy and combination with SAR439684 (PD-1 inhibitor) in patients with advanced solid tumors.
To access the full press release of the 2017 Q2 results, please click here.
2017 Guidance
Sanofi raises full-year 2017 business EPS guidance to broadly stable at CER, barring unforeseen major adverse events. The currency impact on 2017 business EPS is estimated to be approximately +1% at the average June 2017 exchange rates. As announced in the first quarter 2017 financial results, Sanofi previously expected full-year 2017 business EPS to be stable to -3% at CER, barring unforeseen major adverse events.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are generally identified by the words "expects", "anticipates", "believes", "intends", "estimates", "plans" and similar expressions. Although Sanofi's management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, the absence of guarantee that the product candidates if approved will be commercially successful, the future approval and commercial success of therapeutic alternatives, Sanofi's ability to benefit from external growth opportunities and/or obtain regulatory clearances, risks associated with intellectual property and any related pending or future litigation and the ultimate outcome of such litigation, trends in exchange rates and prevailing interest rates, volatile economic conditions, the impact of cost containment initiatives and subsequent changes thereto, the average number of shares outstanding as well as those discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under "Risk Factors" and "Cautionary Statement Regarding Forward-Looking Statements" in Sanofi's annual report on Form 20-F for the year ended December 31, 2016. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.
| Media Relations: |
Investor Relations: |
| Ashleigh Koss |
George Grofik |
| 908-981-8745 |
+33 (0) 1 53 77 45 45 |
| Email: [email protected] |
Email: [email protected] |
SOURCE Sanofi








Share this article